Background
The phase 2 CLL2-BAAG trial tested a triple combination of acalabrutinib (acala), venetoclax (ven) and obinutuzumab (obi) after an optional debulking with bendamustine in patients (pts) with relapsed/refractory (r/r) CLL. In its primary endpoint analysis, 75.6% of pts achieved undetectable MRD (uMRD) in peripheral blood (PB) at final restaging (RE) after approximately 6 months of the triplet (Cramer, Lancet Haematol. 2022). We now report efficacy data and circulating tumor DNA (ctDNA) analyses with additional follow-up.
Methods
In the induction phase, obi was started in cycle 1 (days 1, 8, 15) and given 4-weekly in cycles 2-6. Acala was added in cycle 2 and ven in cycle 3. Maintenance treatment with continuous acala and ven and 3-monthly obi was administered until achievement of a deep remission with uMRD <10 -4 in PB or for up to 24 months (mo). MRD was measured centrally by flow cytometry (FCM) in PB and by digital droplet PCR (ddPCR) of patient-specific VDJ rearrangements and CLL-related mutations in blood plasma. uMRD was defined as <1 CLL cell/10,000 leukocytes (<10 -4) and no detected ctDNA.
Results
In total, 46 pts were included in the trial, 1 pt had to be excluded from the analysis due to a retrospectively noted violation of exclusion criteria. The median number of previous treatments was 1 (range 1-4) and 18 pts (40%) had received a BTK inhibitor (BTKi) and/or ven (BTKi: 8, ven: 7, both: 3) prior to inclusion, 14/44 pts (31.8%) had del(17p) and/or TP53 mutations, 34 (75.6%) had unmutated IGHV.
With a median observation time of 34.4 mo (range 12.0-39.2), all 45 pts are off study treatment. The median treatment duration was 14.7 mo (range 6.1-32.9), 2 pts (4.4%) discontinued treatment during the first 6 induction cycles, both due to adverse events (AEs). Following the MRD- and response-guided approach, 25 pts (55.6%) discontinued therapy according to confirmed uMRD and 9 pts (20.0%) completed the maximum of 8 maintenance cycles due to persisting MRD and/or lack of a complete response.
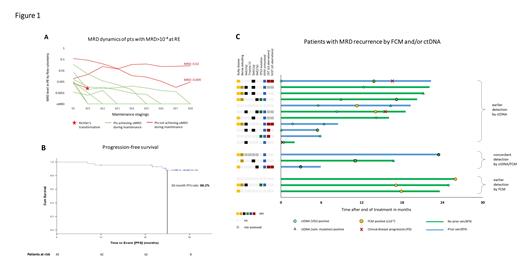
As reported, the uMRD rate in PB at RE was 75.6% with 10 pts showing detectable MRD ≥10 -4. In the course of maintenance, 7 of these 10 pts (70%) achieved uMRD, 1 pt had a Richter's transformation (RT) and 2 pts still had detectable MRD after 8 cycles of maintenance ( Figure 1A).
With all pts off-treatment, uMRD <10 -4 in PB was achieved in 42 of 45 pts (93.3%) at any time point,including 17 of 18 pts (94.4%) previously exposed to ven and/or a BTKi and 13 of 14 pts (92.9%) with TP53 aberrations. The median time to uMRD in PB was 5.4 months from the start of study treatment.
Median progression-free survival (PFS) was not reached, the estimated 30-mo PFS rate for the whole analysis population was 88.2% ( Figure 1B). A similar PFS was shown for pts with TP53 aberrations (30-mo PFS 85.7%) and pts previously exposed to BTKi and/or ven (30-mo PFS 93.3%). The estimated overall survival at 30 mo was 100%. One patient died of COVID-19 18 mo after the end of study treatment.
In total, 564 paired FCM/ctDNA samples were available. Overall, 17 MRD recurrences (after uMRD by both methods) occurred. Of these, 11 were first detected by ctDNA, and 3 by FCM (+3 with concordant detection, Figure 1C). Among those with earlier ctDNA detection, more pts appeared to have high-risk genetics (unmutated IGHV in 10/11 pts, complex karyotype in 4/8 evaluated pts), del(11q) and prior exposure to ven/BTKi compared to those with earlier detection by FCM, and MRD recurrences seemed to occur earlier (mean, 7.1 mo after end of treatment). Notably, in 2 of 3 CLL-type progressions (PD, both nodal with normal lymphocyte counts), ctDNA was detected 6 mo/14 mo before MRD by FCM and 7 mo/17 mo before clinical PD, in the third pt ctDNA and FCM MRD recurred at the same time. In a pt with RT (DLBCL), patient-specific VDJ ctDNA was detected at diagnosis while FCM MRD remained negative.
At the time of the data cut (02/2023) 50 serious AEs (SAEs) were reported, 33 were of CTC G3, 6 of CTC G4 and 1 CTC G5. The most common SAEs were COVID-19 (n=8), other pneumonias (n=5), infusion-related reactions (n=5) and neutropenias (n=3).
Conclusions
Time-limited MRD-guided acala, ven and obi led to an estimated 30-mo PFS rate of 88.2% in pts with r/r CLL. Remissions deepen with ongoing maintenance and almost all pts achieve uMRD in PB including pts with TP53 aberrations and those treated with BTKi and/or ven in prior lines. The addition of ctDNA-based analyses to conventional MRD assessment by FCM seems to improve early detection of relapses.
OffLabel Disclosure:
Furstenau:Roche: Research Funding; Janssen: Research Funding; AstraZeneca: Research Funding; BeiGene: Research Funding; Abbvie: Honoraria, Research Funding. Fink:Abbvie: Other: travel support; AstraZeneca: Consultancy, Honoraria, Research Funding. Weiss:Qiagen: Current Employment. Schneider:Abbvie: Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; BeiGene: Other: travel support; Jannsen Cilag: Consultancy. Tausch:Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; Abbvie: Consultancy, Honoraria, Other: Travel Support, Research Funding, Speakers Bureau; BeiGene: Consultancy, Other: Travel support, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Other: travel support, Speakers Bureau. Fischer:AstraZeneca: Consultancy; Abbvie: Honoraria, Other: TRavel support; Roche: Honoraria, Other: Travel Support. Langerbeins:Abbvie: Honoraria, Other: travel support; Beigene: Honoraria, Other: travel support; Janssen: Honoraria, Other: travel support, Research Funding; AstraZeneca: Honoraria, Other: travel support. Al-Sawaf:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eli Lilly: Speakers Bureau; BeiGene: Research Funding, Speakers Bureau; Adaptive: Speakers Bureau; Ascentage: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Schetelig:Abbvie: Consultancy, Honoraria; Novartis: Honoraria; BeiGene: Consultancy, Honoraria; Eurocept: Honoraria; AstraZeneca: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; BMS: Consultancy, Honoraria. Dreger:Miltenyi: Consultancy; Novartis: Consultancy, Honoraria; bluebird bio: Consultancy; Janssen: Honoraria; Gilead: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Riemser: Honoraria; MD Kompetenz-Centrum Onkologie: Honoraria. Böttcher:Abbvie: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; AstraZeneca: Honoraria, Speakers Bureau; Janssen: Honoraria, Research Funding, Speakers Bureau; Roche: Honoraria, Speakers Bureau. Kreuzer:Abbvie: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau. Ritgen:AstraZeneca: Consultancy, Honoraria, Other: travel support; Roche: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Research Funding; Janssen: Consultancy, Honoraria. Brüggemann:Pfizer: Speakers Bureau; Janssen: Speakers Bureau; Regeneron: Research Funding; BD: Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees; Affimed: Research Funding. Stilgenbauer:Amgen: Consultancy, Honoraria, Other: travel support, Research Funding; Abbvie: Consultancy, Honoraria, Other: travel support, Research Funding; Celgene: Consultancy, Honoraria, Other: travel support, Research Funding; Gilead: Consultancy, Honoraria, Other: travel support, Research Funding; GSK: Consultancy, Honoraria, Other: travel support, Research Funding; Roche: Consultancy, Honoraria, Other: travel support, Research Funding; Janssen: Consultancy, Honoraria, Other: travel support, Research Funding; Novartis: Consultancy, Honoraria, Other: travel support, Research Funding; Sunesis: Consultancy, Honoraria, Other: travel support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: travel support, Research Funding. Eichhorst:F. Hoffmann-La Roche Ltd: Honoraria, Research Funding, Speakers Bureau; MSD: Consultancy, Honoraria, Speakers Bureau; Lilly: Consultancy, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Gilead: Consultancy, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Abbvie: Consultancy, Honoraria, Research Funding, Speakers Bureau. Hallek:Gilead: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding. Cramer:Novartis: Research Funding; Gilead: Research Funding; BeiGene: Consultancy, Other: travel support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: travel support, Research Funding; Janssen: Honoraria, Research Funding; Acerta: Research Funding; Roche: Honoraria, Other: travel support, Research Funding; Abbvie: Consultancy, Honoraria, Other: travel support, Research Funding; BMS: Honoraria.
The combination of acalabrutinib, venetoclax, obinutuzumab is not approved for the treatment of CLL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal